
 Divide the moles of solute found in Step 1 by the liters of solvent found in Step 2 to find the initial concentration of a solution. Measure the amount of the solvent that you have. Weigh the amount of solute (the compound being dissolved) in grams. The Maximum Acceptable Concentration (MAC) of Pb in drinking water is 10 ppb. To convert from molarity to mg/L (or ppm in dilute solution), multiply by the molar mass of the analyte to convert moles into corresponding number of moles. 1% m/v solutions are sometimes thought of as being gram/100 mL but this detracts from the fact that % m/v is g/mL 1 g of water has a volume of approximately 1 mL (at standard temperature and pressure) and the mass concentration is said to be 100%. Thus 100 mL of water is equal to approximately 100 g. What are three ways to measure the concentration of a solution? Concentration can be expressed as percent by volume, percent by mass, and molarity. What are 3 ways to measure the concentration of a solution? More: ppb (or ppbm) is used to measure the concentration of a contaminant in soils and sediments. PPM to Molarity Convert ppm to gram based or milligram based concentration.
Divide the moles of solute found in Step 1 by the liters of solvent found in Step 2 to find the initial concentration of a solution. Measure the amount of the solvent that you have. Weigh the amount of solute (the compound being dissolved) in grams. The Maximum Acceptable Concentration (MAC) of Pb in drinking water is 10 ppb. To convert from molarity to mg/L (or ppm in dilute solution), multiply by the molar mass of the analyte to convert moles into corresponding number of moles. 1% m/v solutions are sometimes thought of as being gram/100 mL but this detracts from the fact that % m/v is g/mL 1 g of water has a volume of approximately 1 mL (at standard temperature and pressure) and the mass concentration is said to be 100%. Thus 100 mL of water is equal to approximately 100 g. What are three ways to measure the concentration of a solution? Concentration can be expressed as percent by volume, percent by mass, and molarity. What are 3 ways to measure the concentration of a solution? More: ppb (or ppbm) is used to measure the concentration of a contaminant in soils and sediments. PPM to Molarity Convert ppm to gram based or milligram based concentration. 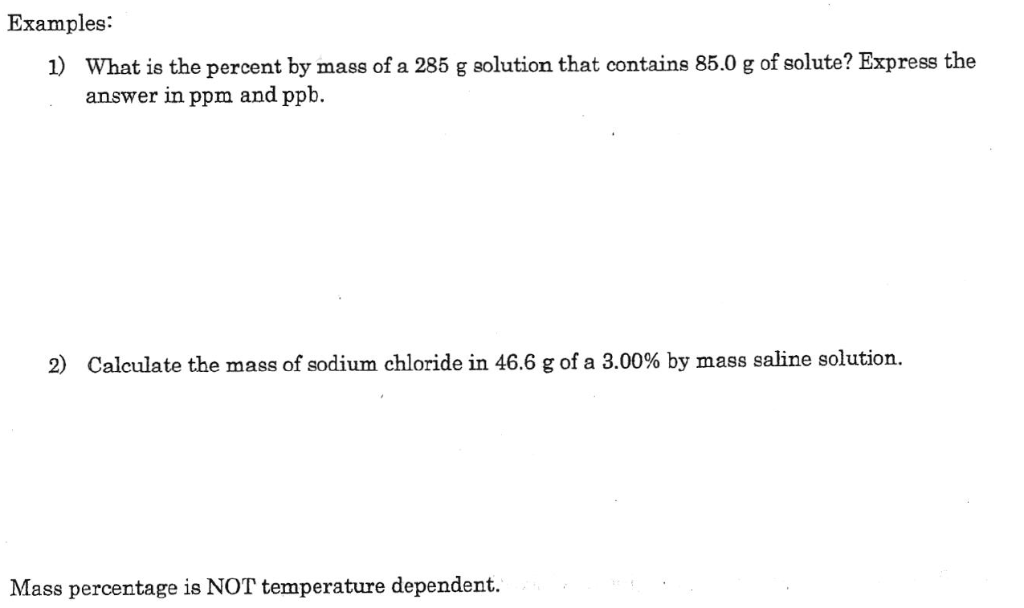
Go here for ISE molarity/ppm conversions shown in Table III. Fluoride has a FW of 19, hence a 10-3 M concentration is equal to 19ppm, 1M is equal to 19,000 ppm and 1ppm is equal to 5.2 x 10-5 M.

Parts per billion (ppb) is the number of units of mass of a contaminant per 1000 million units of total mass. One part per million (ppm) denotes one part per 1,000,000 parts, one part in 106, and a value of 1 × 106. The FW of an ion species is equal to its concentration in ppm at 10-3 M. In the case of a mass ratio of 0.000005, this would give 5,000 ppb. Calculate the concentration in ppb by multiplying the ratio of the mass of solute to mass of solution by 1 billion, or 1,000,000,000.







 0 kommentar(er)
0 kommentar(er)
